EN
Language
All categories
Product ID: 68589862
Disposable Surgical Gown
Contact Now
Description
Basic Info.
- Model NO. VGW01
- Ethylene Oxide Sterilization Ethylene Oxide Sterilization
- Quality Guarantee Period Two Years
- Group Adult
- Logo Printing Without Logo Printing
- Color White/Blue/Green/Pink/Yellow
- Usage Safety & Protective
- Package 10PCS/Bag, 100PCS/CTN
- Model No Vgw01
- Transport Package Carton
- Specification S-6XL
- Trademark OEM/VMED
- Origin China
- HS Code 6210103000
- Production Capacity 30000PCS/Day
Product Description
Specification of SMS Surgical gown
- Material SMS20-60G/m2
- Logo Printing Without Logo Printing
- Trademark VEMD
- Color White/Blue/Green
- Style Knitted cuffs/Extra long ties sewn at waist and neck/Sewn seams/Latex free
- Size S-6XL, or as required
- Certificate FDA, CE, ISO
- Transport Package 1pcs/bag, 50pcs/ctn
- HS Code 6210103000
- Origin China
Protection Level:

Details

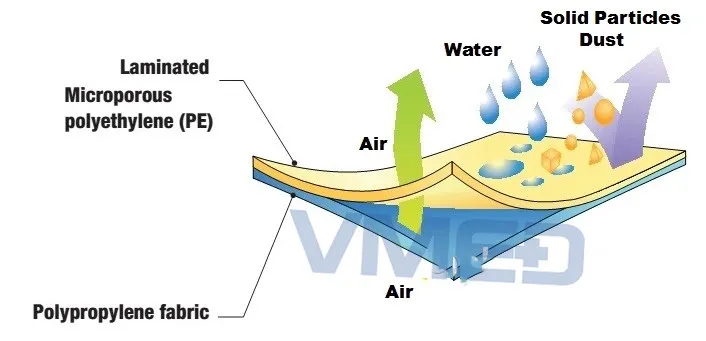
Fabric
| Type | Brief Introduction | Dust proof | Liquid proof |
|---|---|---|---|
| SPP | Via extrusion methods to product melt-blown and spun-bond fiber | 50-70μm (35-45gsm) | √ |
| SMS | Spunbond + meltblown + spunbond nonwoven | Yes | √√√ |
| Microporous | Microporous-membrane (barrier coating) + polypropylene nonwoven | 10-30μm (45-55gsm) | √√√√ |
| PP+PE | PP with PE coating | 0.5μm (as Tyvek) | √√ |
Advantage
VMED is committed to providing worldwide customers a safer, healthier experience


Company Information

Product Display



VMED Workmanship -- Quality assurance


FAQ
- Q1: Can I have a sample first? VMED: Of course! Sample are available and free.
- Q2: Do your company have any quality management system such as ISO, EN or CE certificate(s)? VMED: Yes, we have CE, ISO13485, FDA,
- Q3: What's your advantage? Why we choose you? VMED: Vench Medical Products Co., Ltd. is a fast growing company, specializing in the manufacture of disposable medical products and personal protection products. Our company has implemented a strict quality management system (QMS) and has passed ISO13485 certification. Our main products have passed CE certification of European Union (EU) and FDA registration of USA.
- Q4: How does your factory control the quality? VMED: All manufacturing are absolutely base on the pre-production samples. Meanwhile, we have a complete after-sales system.
- Q5: What the expiration date for disposable non-woven products? VMED: We suggest 2 years of shelf storage.
Trust us -- professional & safety
Contact Now
Please Send Message
${currentPro.title}

















